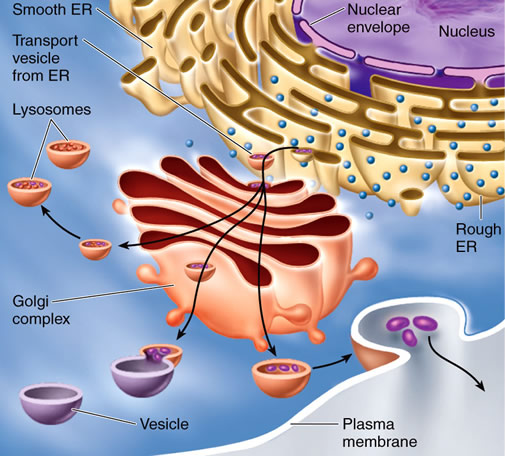
The endomembrane system is a collective term applied to all of the membranes in a cell that are either connected with or are derived from the endoplasmic reticulum (ER), including the plasma membrane but not the membranes of chloroplasts or mitochondria.
The membrane-bound organelles considered to be part of the endomembrane system are the vacuole, nuclear envelope, endoplasmic reticulum, Golgi complex, and various types of vacuoles.
Some components of the endomembrane system have direct, permanent connections with the endomembrane system (such as between the endoplasmic reticulum and the nuclear envelope), whereas other components share membrane and contents by trafficking vesicles (membrane-bound packages) from one component to another (for example, the ER sends numerous vesicles to the Golgi complex) across the cytosol.
  |
The endomembrane system is responsible for processing, sorting, and packaging membrane material, proteins embedded in membranes, and large water-soluble molecules (such as proteins or carbohydrates), either for export from the cell (called exocytosis) or for use within the cell. The endoplasmic reticulum is the ultimate source of all the membranes of the endomembrane system.
Golgi Complex
The Golgi complex is a major component of the endomembrane system and, in most cells, its primary role is secretion. The term “Golgi complex” refers collectively to all the Golgi bodies (once commonly called dictyosomes in plants) in a cell.
It is named after Camillo Golgi (1843-1926), an Italian scientist who first described the structures in 1878. Golgi won the 1906 Nobel Prize in Physiology or Medicine for his contributions to the understanding of the structure of the nervous system.
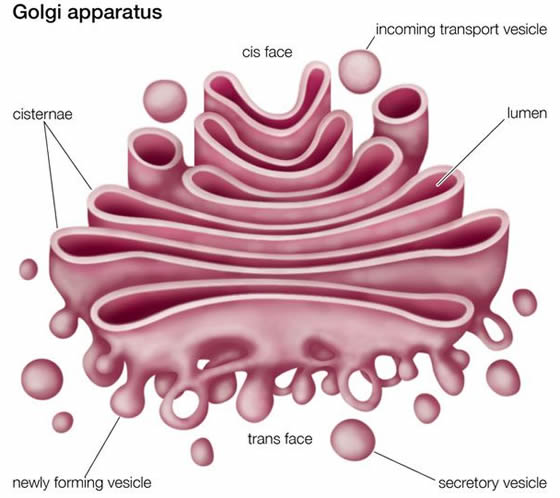
When viewed through an electron microscope, a single Golgi body is composed of a series (of typically four to eight) round, flattened membranous sacs called cisternae.
This “stack of sacs” has two sides; the cisterna on the cis side often faces the ER, while the cisterna on the trans side often faces away from the ER. The medial cisternae are in between. The trans-Golgi network is the collection of vesicles seen leaving the trans face of the Golgi body.
Intercisternal elements are protein fibers that span the space between cisternae. They may help anchor the Golgi enzymes in the individual cisternae so they are not transported away and lost with the shuttle vesicles, described below. The intercisternal elements may also help stabilize the entire Golgi body.
 |
| Golgi complex |
An individual Golgi body is surrounded by a very faint, filamentous structure called the Golgi matrix. The matrix (along with the intercisternal elements) probably helps hold the Golgi body together as it is moved around the cell.
The matrix also appears to exclude ribosomes and other cytosolic components from the immediate vicinity of the Golgi bodies and keeps the cytoplasm from interfering with the functioning of the complex. There are apparently no direct membrane connections among the individual cisternae in a Golgi body.
Rather, membrane and contents move between cisternae via shuttle vesicles that pinch off of one cisterna, move in a trans direction, and fuse with an adjacent cisterna. The number of Golgi bodies in a plant cell can vary widely, from several dozen to more than ten thousand, depending on the size and function of the cell.
Entire Golgi bodies are transported around the cell via the cytoskeleton. They showa stop-and-go type of movement that may be associated with a need to approach a segment of the rough ER, or RER, to pick up proteins and then move closer to the plasma membrane or vacuole to deliver those proteins to the proper destination. Golgi bodies divide by fission, with new stacks pinching off of existing ones.
   |
Golgi Functioning
The Golgi complex is responsible for the processing and packaging of proteins and other molecules for secretion from the cell or use within the cell. A typical pathway for the secretion of a protein to the cell exterior (that is, exocytosis) would be as follows.
A ribosome bound to the RER translates a piece of messenger RNA into a protein, and that protein is inserted into the lumen (the aqueous space enclosed by the RER membrane) of the RER. A vesicle forms by enclosing the protein and pinching off of the RER.
This transition vesicle travels across the cytosol (the traffic being directed by the microtubules and microfilaments of the cytoskeleton), fuses with the cisterna on the cis face of a Golgi body, and delivers the protein to the lumen of that cisterna.
Enzymes in the lumen of the cisterna modify the protein by adding sugars (called “glycosylation”) to produce a glycoprotein. A vesicle of Golgi membrane pinches off, forms a shuttle vesicle, and delivers the glycoprotein to the next cisterna in the stack, where the protein may be further glycosylated.
The protein moves its way through the Golgi body and, eventually, leaves the trans face in a secretory vesicle that fuses with the plasma membrane. The contents of the secretory vesicle are moved out of the cell by exocytosis, while the membrane of the vesicle becomes incorporated into the plasma membrane.
In a similar fashion, the Golgi complex delivers matrix polysaccharides to the cell wall, large molecules and membrane lipids to the vacuole, or integral membrane proteins to the vacuolar membrane.
In contrast to proteins (which are synthesized on the RER and merely modified during their transit through the Golgi body), cell wall matrix polysaccharides are synthesized from the ground up in the Golgi.
Surface-exposed proteins in the membrane of the vesicle contain the information needed to direct the vesicle to either the plasma membrane or some other place in the cell.
Vesicles destined for the vacuole are visibly coated (as seen using an electron microscope) with protein, the major coat protein being clathrin. Vesicles destined for the plasma membrane appear smooth but undoubtedly have protein information on the exterior that directs them to the plasma membrane.
A single Golgi body can be involved in processing and packaging both glycoproteins and polysaccharides for delivery to the plasma membrane or intracellular locations at the same time.
They also can be “retailored” to suit changing needs over the lifetime of a cell. That is to say, the enzymes in the cisternal lumen can be degraded and replaced with other enzymes that direct the synthesis of different molecules.
Other Endomembrane System Components
From the above, it can be seen how the ER and Golgi complex interact to deliver proteins and carbohydrates to the plasma membrane and vacuoles.
In addition, the electron microscope often shows the ER to be physically connected to the nuclear envelope. Thus, the nuclear envelope is almost always included in a discussion of the endomembrane system. However, the exact functional nature of this nuclear connection remains unknown.
The nuclear envelope is composed of two membranes, the outermost of which can be studded with ribosomes, much like the RER. The outer membrane of the nuclear envelope may, in some ways, be functionally similar to the RER, producing proteins which are passed across the outer membrane to the space between the two envelope membranes.
The fate of those membranes remains unclear. Whether they cross the inner membrane of the nuclear envelope aswell and are delivered to the interior of the nucleus or diffuse to the lumen of a nearby section of ER and are processed through the Golgi complex is not known. The ER/nuclear envelope connection may be better understood by investigating the evolution of the eukaryotic cell.
Evolutionary Significance
According to the endosymbiotic theory, the ancestor of today’s eukaryotic cells was a primitive prokaryote that engulfed a respiratory prokaryote (which eventually became established as mitochondria) and a photosynthetic prokaryote (which became chloroplasts).
The other membrane-bound organelles of the cytoplasm were derived from infoldings of the plasma membrane. Thus the entire endomembrane system (plasma membrane, ER, Golgi complex, vacuoles, and nuclear envelope) probably has a common evolutionary background. Over time, the individual compartments became more specialized.