 |
| Carbon Cycle |
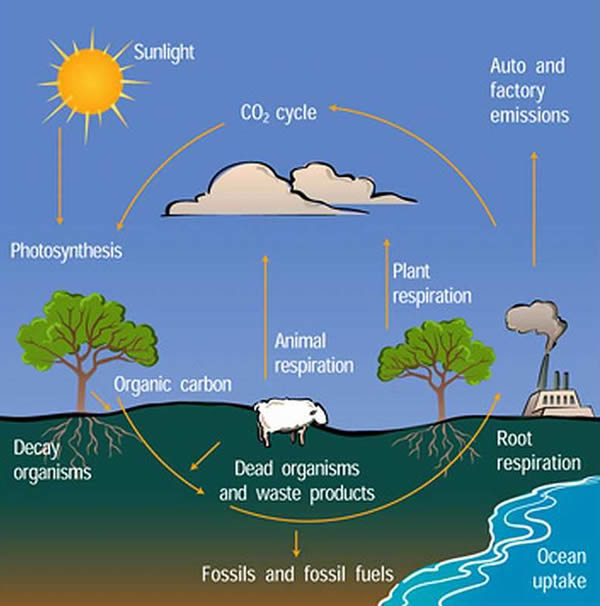
The carbon cycle is the movement of the element carbon through the earth’s rock and sediment, the aquatic environment, land environments, and the atmosphere. Large amounts of organic carbon can be found in both living organisms and dead organic material.
An enormous reservoir of carbon, on the order of 20 x 1015 tons, may be found on the surface of the earth. Most of this reservoir is found in rock and sediment. The carbon cycle therefore represents the movement of this element through the biosphere in a process mediated by photosynthetic plants on land and in the sea.
The process involves the fixation of carbon dioxide (CO2) into organic molecules, a process called photosynthesis. Energy used in the process is stored in chemical form, such as that in carbohydrates (sugars such as glucose). The organic material is eventually oxidized, as occurs when a photosynthetic organism dies.
  |
Through the process of respiration, the carbon is returned to the atmosphere in the form of carbon dioxide. Because the “turnover” time of such forms of carbon is so slow (on the order of thousands of years), the entrance of this material into the carbon cycle is insignificant on the human scale.
Photosynthesis
Organisms that use carbon dioxide as their source of carbon are known as autotrophs. Many of these organisms also use sunlight as the source of energy for reduction of carbon dioxide; hence, they are frequently referred to as photoautotrophs. This process of carbon dioxide fixation is carried out by phytoplankton in the seas, by land plants (particularly trees), and by many microorganisms. Most of the process is carried out by the land plants.
The process of photosynthesis can be summarized by the following equation:
CO2 + water + energy → carbohydrates + oxygen
The process requires energy from sunlight, which is stored in the form of the chemical energy in carbohydrates. While most plants produce oxygen in the process—the source of the oxygen in the earth’s atmosphere—some bacteria may produce products other than oxygen. Organisms that carry out carbon dioxide fixation, using photosynthesis to synthesize carbohydrates, are often referred to as producers.
Approximately 20 billion to 30 billion tons of carbon are fixed each year by the process—clearly a large amount but only a small proportion of the total carbon found on the earth. Approximately 450 billion tons of carbon are contained within the earth’s forests; some 700 billion tons exist in the form of atmospheric carbon dioxide.
Much of the organic carbon on the earth is found in the form of land plants, including forests and grasslands. When these plants or plant materials die, as when leaves fall to the earth in autumn, the dead organic material becomes humus. Much of the carbon initially bound during photosynthesis is in the form of humus.
Degradation of humus is a slow process, on the order of decades. However, it is the decomposition of humus, particularly through the process called respiration, that returns much of the carbon dioxide to the atmosphere. Thus, the carbon cycle represents a dynamic equilibrium between the carbon in the atmosphere and carbon fixed in the form of organic material.
Respiration
Respiration represents the reverse of photosynthesis. All organisms that use oxygen, including humans, carry out the process. However, it is primarily humic decomposition by microorganisms that returns most of the carbon to the atmosphere. Depending on the particular microorganism, the carbon is in the form of either carbon dioxide or methane (CH4). Respiration is generally represented by the equation:
Carbohydrate + oxygen → carbon dioxide + water + energy
Energy released by the reaction is used by the organism (that is, the consumer) to carry out its own metabolic processes.
Carbon Sediment
 |
| Carbon sediment |
Despite the enormous levels of carbon cycled between the atmosphere and living organisms, most carbon is found within carbonate deposits on land and in ocean sediments. Some of this originates in marine ecosystems, where organisms use dissolved carbon dioxide to produce carbonate shells (calcium carbonate).
As these organisms die, the shells sink and become part of the ocean sediment. Other organic deposits, such as oil and coal, originate from fossil deposits of dead organic material. The recycling time for such sediments and deposits is generally on the order of thousands of years; hence their contribution to the carbon cycle is negligible on a human time scale.
Some of the sediment is recycled naturally, as when sediment dissolves or when acid rain falls on carbonate rock (limestone), releasing carbon dioxide. However, when such deposits are burned as fossil fuels, the levels of carbon dioxide in the atmosphere may increase at a rapid rate.
Environmental Impact of Human Activities
Carbon dioxide gas is only a small proportion (0.036 percent) of the volume of the atmosphere. However, because of its ability to stat, and even small changes in levels of this gas can significantly alter environmental temperatures. Around 1850, humans began burning large quantities of fossil fuels; the use of such fuels accelerated significantly after the invention of the automobile.
By the end of the twentieth century, between 5 billion and 6 billion tons of carbon were being released into the atmosphere every year from the burning of fossil carbon. Some of the released carbon probably returns to the earth through biological carbon fix a trap heat from the earth, carbon dioxide acts much like a thermostat, and even small changes in levels of this gas can significantly alter environmental temperatures.
Around 1850, humans began burning large quantities of fossil fuels; the use of such fuels accelerated significantly after the invention of the automobile. By the end of the twentieth century, between 5 billion and 6 billion tons of carbon were being released into the atmosphere every year from the burning of fossil carbon.
Some of the released carbon probably returns to the earth through biological carbon fixation, with a possible increase in the land biomass of trees or other plants. (Whether this is so remains a matter of dispute.) Indeed, large-scale deforestation could potentially remove this means by which levels of atmospheric carbon dioxide could be naturally controlled.